

They are each at thermal equilibrium, but at different temperatures. We have two identical blocks of metal, say aluminum. The isolated system is like a little universe all to itself In an isolated system nothing gets in or out, neither heat nor mass nor even any radiation like light. If the second law of thermodynamics contributed to evolution, life in general could not exist over this amount of time because the energy would decrease constantly until none was left.An isolated system is a little more than just adiabatic. Living things have mandatory needs in order to live including energy. The second law of thermodynamics can portray a contradictory perspective of evolution but the source of energy must be considered. The amount of useful energy does not decrease among living things but is recycled. Bodies of all organisms use the same basic types of molecules therefore can evolve without loss of energy. They can break but the energy (protons, electrons) goes elsewhere to another molecule and bond. All chemical bonds have energy that cannot be broken down. Water molecules including other molecules contain chemical bonds. Without cohesion, plants would not have been able to survive. This enables plants to retain water molecules that are chained.

Water molecules have high cohesion that produces surface tension. To maintain orderly structures, living things use this energy to synthesize complex molecules.Ī specific point to prove the theory of evolution is cohesion of water and molecules. The sun produces concentrated energy, which increases entropy.
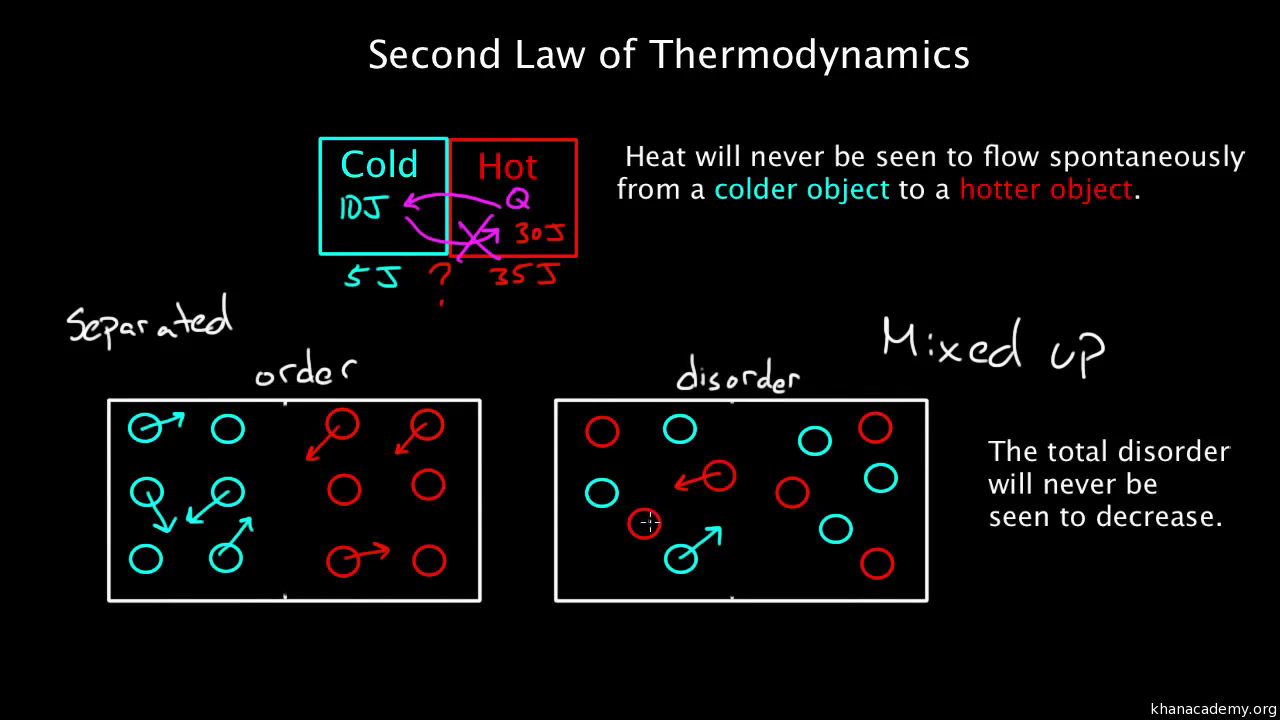
The second law of thermodynamics is not relating to energy within living things because living things retain energy from the sun. In relation to the theory of evolution, this law is not contradictory. EvolutionĪccording to the second law of thermodynamics, when energy is converted from one form to another, the amount of useful energy decreases.


 0 kommentar(er)
0 kommentar(er)
